Intelligent Spine Interface
Radu Darie , Jonathan Calvert , Samuel Parker
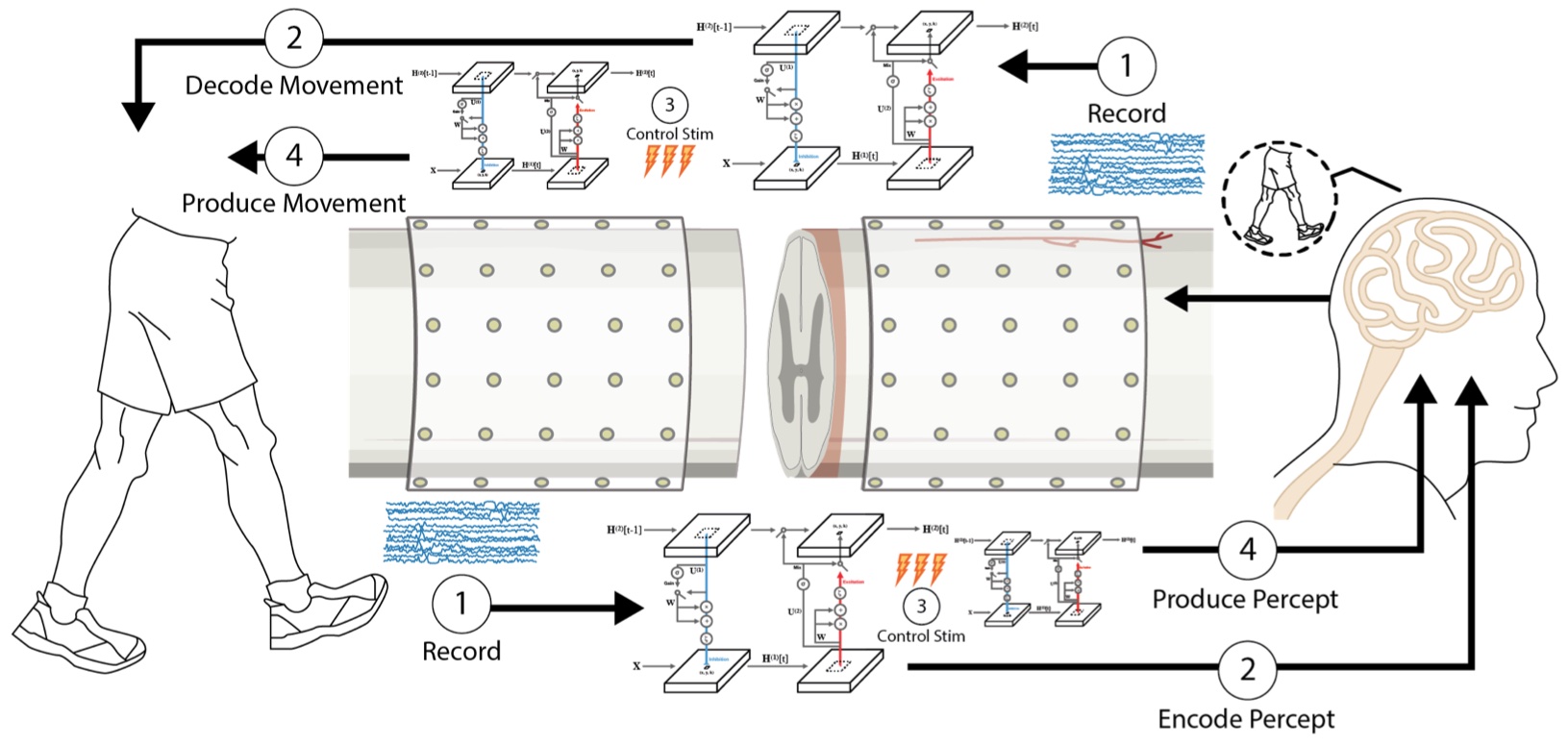
At present, activity-based therapies are the only medical practices that can be used to enhance recovery after spinal cord injury (SCI). However, the most affected patients, who fail to produce active movements voluntarily, experience minimal benefits from such therapies. Several studies have now shown that spinal cord stimulation delivered at the right time can enhance a physical therapy rehabilitation program significantly, leading to restoration of volitional walking (when the stimulator is on). We therefore propose a bi-directional tool for sensing and stimulating to bridge a gap in the spinal cord, ‘reconnecting’ patches of eloquent nervous system tissue. The Intelligent Spine Interface (ISI) will be capable of reading and writing simultaneously to, and from, the human spinal cord both above, and below, the site of SCI. The ISI will interpret neural information from above a spinal cord lesion and transfer that information, via state-of-the-art artificial neural network-based interpreters, to sites below the lesion and restore volitional control of the lower limb. We will focus our therapeutic demonstrations on the restoration of bi-directional sensing and control of the legs and voluntary locomotion. The proposed technology is agnostic to the level of the spinal lesion and has far greater number of sites (electrodes) to interact with the nervous system, thus making the therapeutic potential far greater than current technologies.
Related Publications:
3D Multicellular Models of the Brain
Sophie Brown , Abby Drexler | Collaborators: Huang Lab
As the most common form of neurodegenerative disease, Alzheimer Disease (AD) contributes to nearly 60–70% of all cases of dementia globally. Currently, the path towards successful therapeutic intervention is obstructed by a limited understanding of disease etiology and the myriad of proposed underlying mechanisms. Evidence indicating increased levels of inflammatory markers in AD patients, as well as immune-associated loci of several AD risk genes, suggests that neuroinflammation is a contributing factor to pathogenesis. As microglia are dynamic mediators of neuroinflammation, determining their specific roles in AD pathology is essential to deciphering neuroinflammatory influence on underlying AD mechanisms. Here in the Borton Lab we are working to develop a hybrid in vitro model to study human iPSC-derived microglia (iMGs) in a 3D xenoculture system. We hypothesize that hybrid 3D xenocultures can be utilized to study human microglial behaviors that may contribute to AD pathogenesis in a physiologically-relevant in vitro environment. Our overall objectives in this research program are to (1) develop novel in vitro tools for studying complex cellular biology in the brain and (2) characterize the roles of human microglia during neuroinflammatory disease states.


Related Publications:
Open Mind Consortium
Bradford Roarr , Evan Dastin van Rijn , Nicole Provenza | Collaborators: UCSF, Mayo Clinic, Oxford
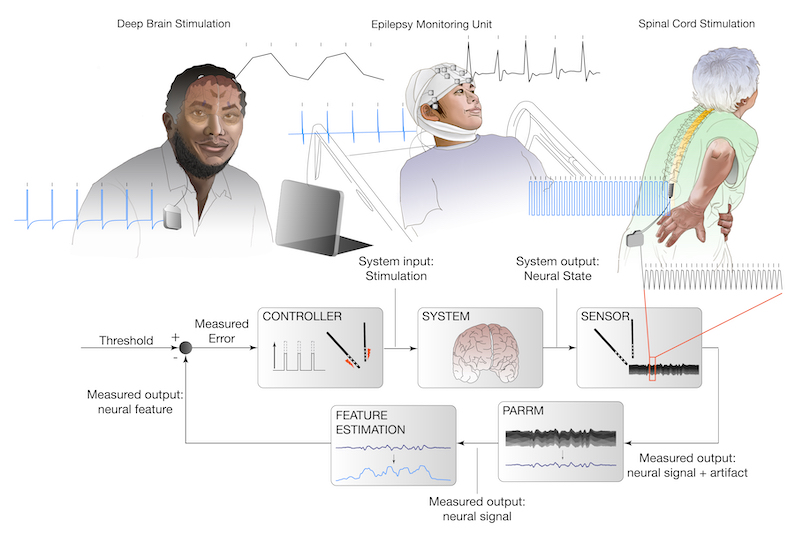
The OpenMind neural communications consortium was formed to accelerate cooperation and innovation in the use of implantable neurostimulation hardware platforms. These next generation devices incorporate sensing of cortical and subcortical field potential activity, with the capability for wireless streaming from the internal device to external computers over years. Our founding team represents the major clinical areas of interest in neuromodulation –– movement disorders (UCSF), epilepsy (Mayo Clinic), and psychiatry (Brown/Baylor), and includes experts in the design and dissemination of implantable devices (Oxford), and in neuroethics. This consortium will facilitate already funded proposals, as well as entry of new investigators, in the rapidly evolving ecosystem of implantable wireless neural interfaces. Our goal is to provide investigators with critical elements to the launch of their own clinical studies –– A “turnkey” user interface to get started, a library of more sophisticated, open source software elements for neural sensing at home paired with peripheral monitors, and streamlined regulatory pathway for FDA approval of investigational protocols, which we call the “Open Source Quality Management System”. We will disseminate education and resources through biannual workshops and a web-based library of regulatory documents, software, and the Quality Management System.
Related Publications:
Understanding Cortical Control of Locomotion
David Xing
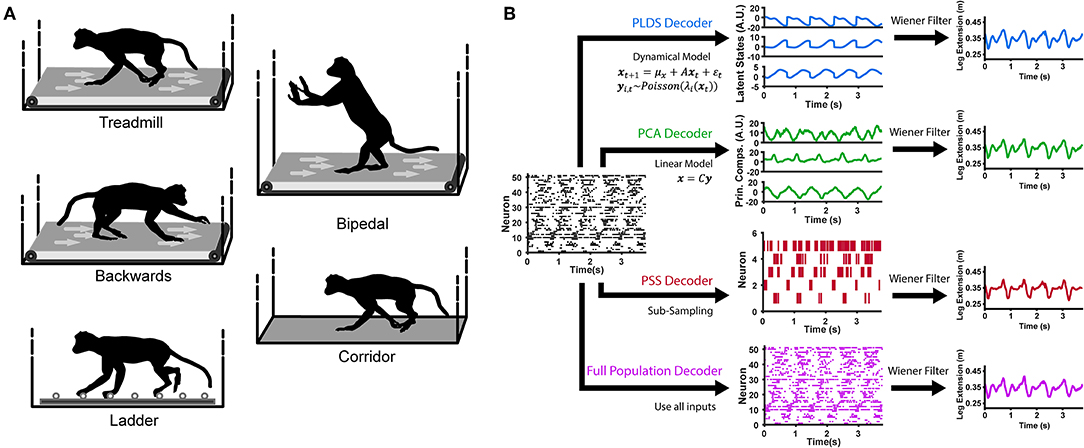
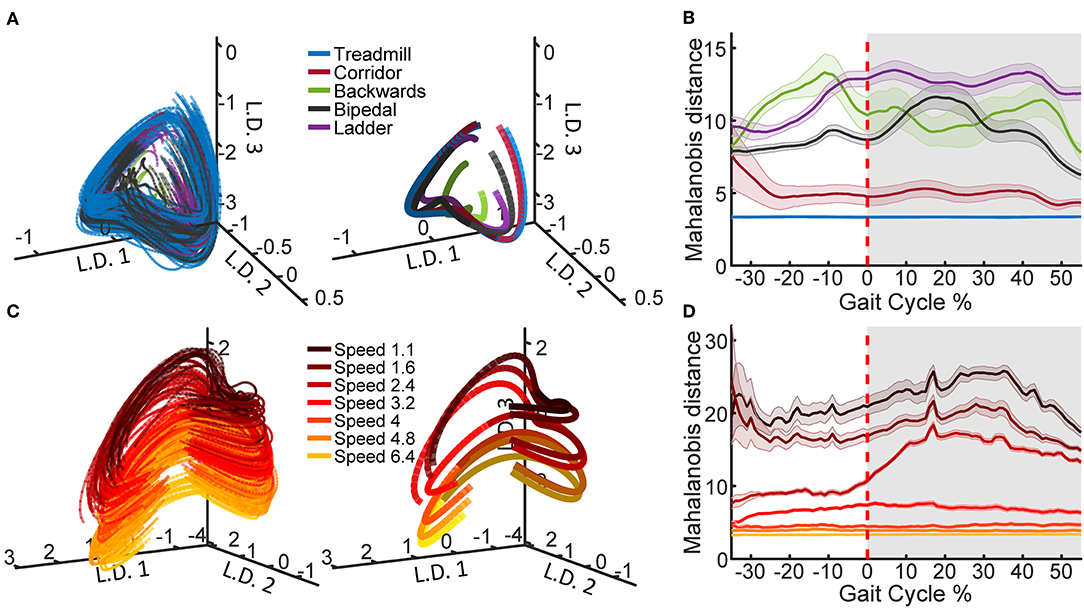
The field of brain-machine interfaces (BMI) for restoring forelimb motor function has made considerable progress over the last few decades. However, the development of hind-limb counterparts have remained relatively nascent. Although there have been recent advancements in restoring basic locomotion in both animal models and in the clinic, the number of developments for neuroprosthetics allowing direct control over voluntary hind-limb movements is still sparse. In this project, we are developing a closed-loop BMI system allowing for direct end-point control of the foot. Using implanted multi-electrode arrays and machine learning techniques, neural signals recorded from motor cortex will be decoded and translated into movement of a robotic actuator in real time. The viability and functionality of our system will be validated in a pedal positioning task. Additionally, in order to integrate both voluntary hind-limb movements with autonomous locomotion into one general-purpose BMI, it is necessary to understand how the motor cortex encodes hind-limb movement during these two behaviors. We employ an obstacle avoidance paradigm to probe the neural correlates and network dynamics of leg-M1 during both voluntary (e.g. originating from cortical areas) and autonomous (e.g. originating from spinal circuits) action. The ultimate goal would be to develop general-purpose, high-functioning neuroprosthetics for the hind-limb allowing for a wide variety of motor actions and enabling patients to regain full lower limb function.
Related Publications:
Proprioprosthetics
Radu Darie | Collaborators: Medtronic
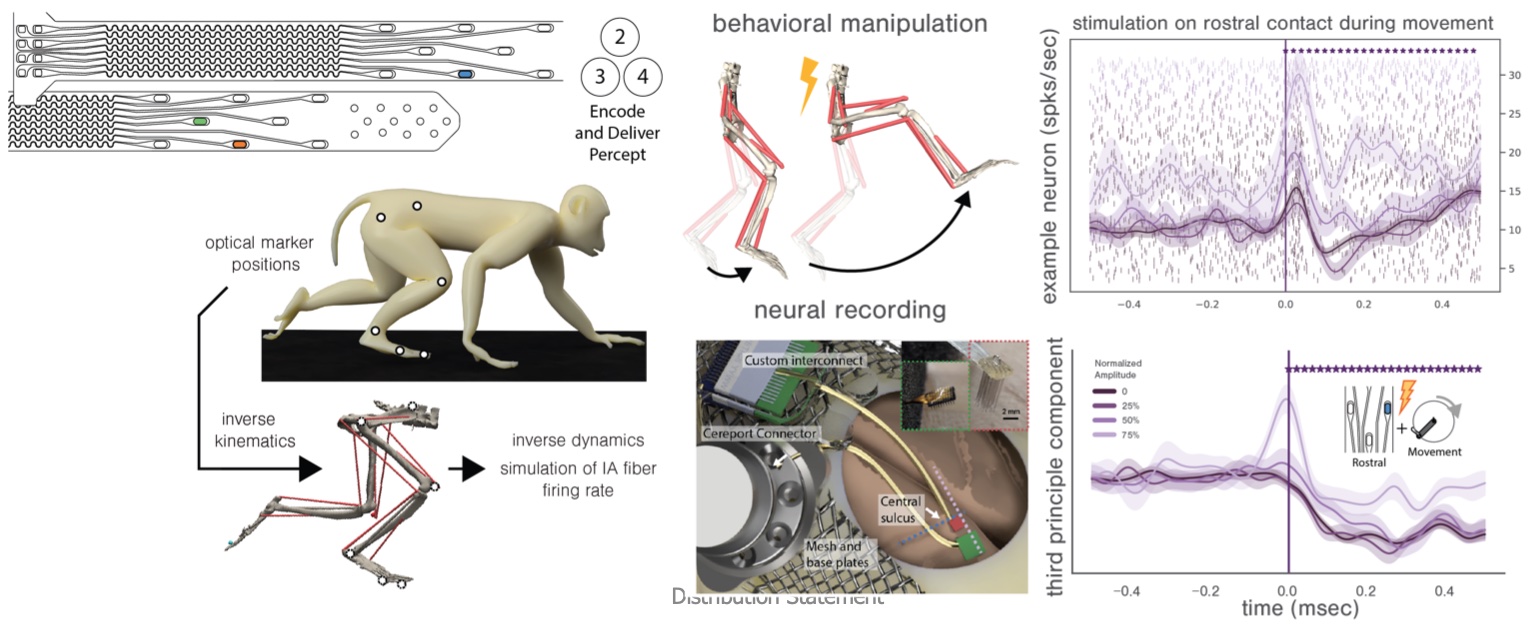
Spinal cord stimulation delivers proprioceptive information to the animal. The effects of that stimulation are observed in the somatosensory cortex. In an intact organism, locomotion and posture are controlled effortlessly and accurately with feedback from proprioceptive somatosensory pathways. The technology we are developing seeks to provide the users of an instrumented prosthetic leg with rich and naturalistic feedback about their prosthesis so that they might move with equal finesse. By mimicking the pattern of neural activation that would be observed from an intact limb and delivering it to the nervous system through epidural spinal cord stimulation, we believe we can make the users perceive the movements of their prosthetic as they would their own limbs. Toward that goal, we are performing an experiment where an intact non-human primate discriminates the magnitude of a passive leg movement.
Related Publications:
Neural Dynamics of Pain Processing in the Spine-Brain Continuum
Christopher Black | Collaborators: Cleveland Clinic
This project focuses on the neural dynamics governing pain processing. Our goal is to develop a mechanistic framework of pain relay in order to optimize chronic pain therapies, such as spinal cord stimulation (SCS). While SCS has been used to treat chronic pain patients, it is unclear how stimulation modulates neural circuits that are responsible for pain perception. Therefore, we are developing biophysically realistic neural models of spinal and cortical circuits implicated in pain processing to examine the effects of electrical stimulation on neural activity. In parallel efforts, we are recording acute and chronic electrophysiology in mice to study spinal and cortical circuits in vivo in response to sensory stimuli.
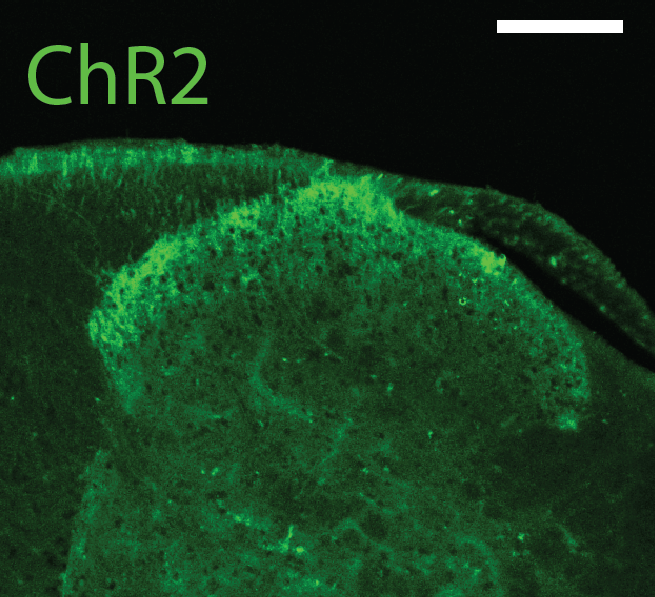
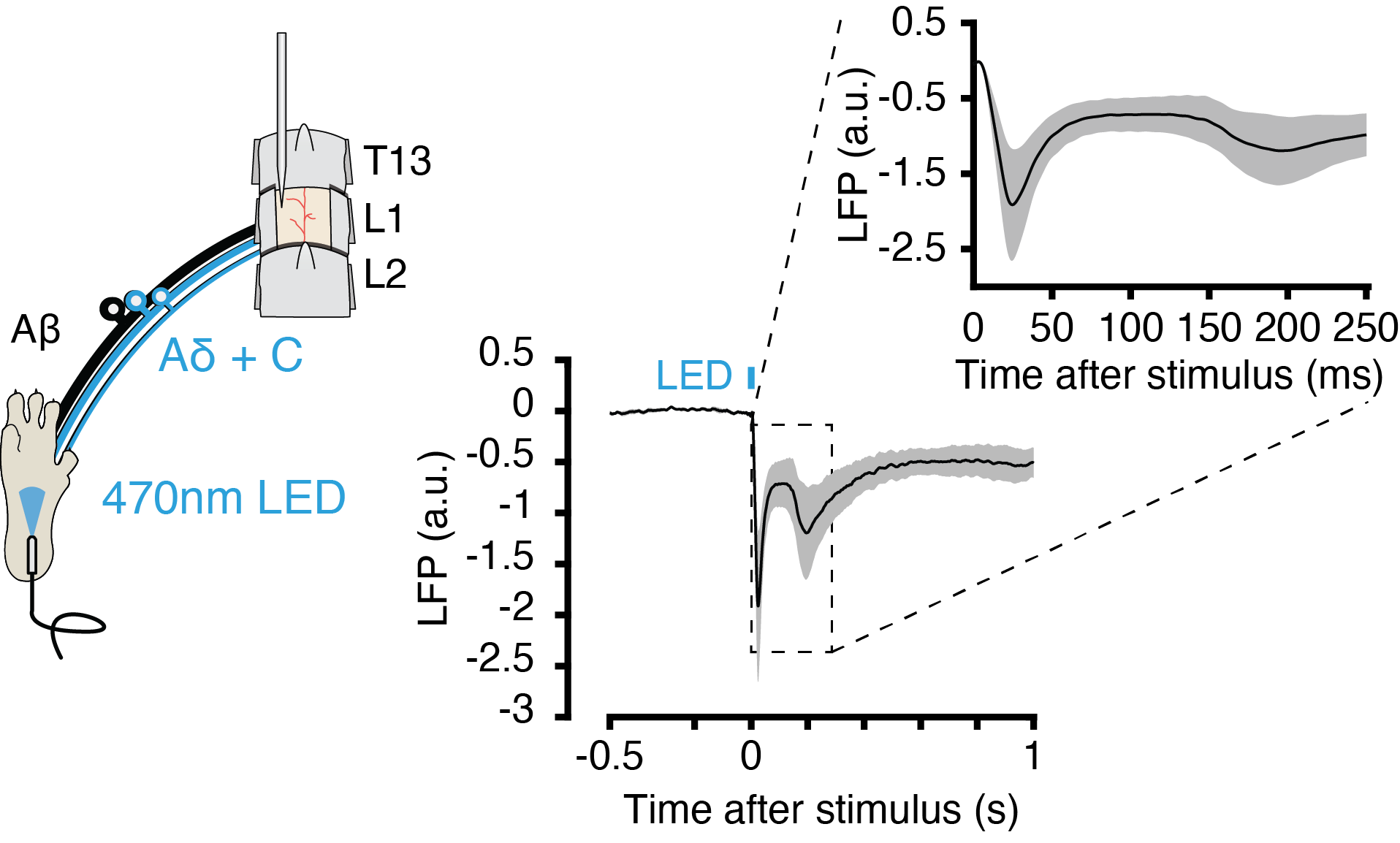
Related Publications:
Corticospinal Neurovascular Dynamics in Health and Disease
Dmitrijs Celinskis , Adriel Barrios-Anderson | Collaborators: Christopher Moore Lab, Cleveland Clinic
Our understanding of the cellular mechanisms underlying the processing of tactile and nociceptive signals in the spinal cord is still in its infancy. In the recent years, several studies have emerged advancing our understanding of these mechanisms by revealing the implicated cellular species. However, despite the value of this new evidence, the up-to-date in vivo studies primarily relied on pharmacogenetic approaches, offering only static information on these intricately dynamic processes. We approach this question of spinal cord information processing in health and disease by employing one- and two-photon imaging in behaving transgenic animals. This allows us to eavesdrop on neurovascular dynamics during various tactile and painful stimuli. Better understanding of these dynamics is of particular interest for clinical pain management. One particular therapeutic intervention of high relevance is electrical spinal cord stimulation. This therapy can offer significant pain relief and is administered in thousands of new patients every year, but comes with significant inter-patient variability and decline of efficacy over time. The origins of these shortcomings remain unknown. To shed some light on the possible mechanisms behind spinal cord stimulation therapy, we employ electrical stimulation using custom-designed spinal probes in conjunction with spinal microscopic imaging. This multi-modal approach enables our unique ability of asking both the basic and clinical neuroscience questions about spinal processing of tactile and painful stimuli under normal and electrically stimulated conditions.
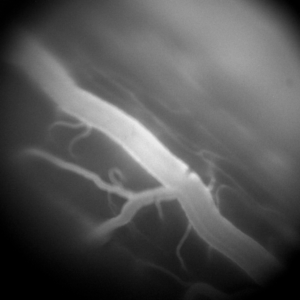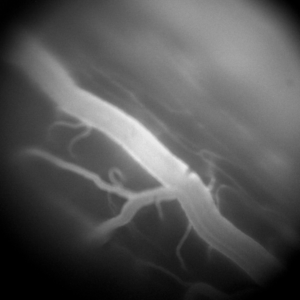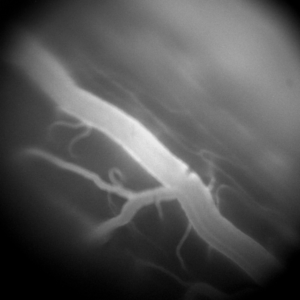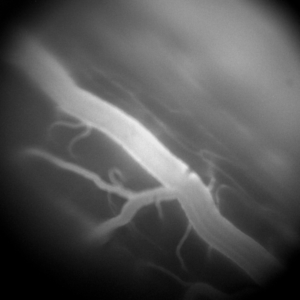
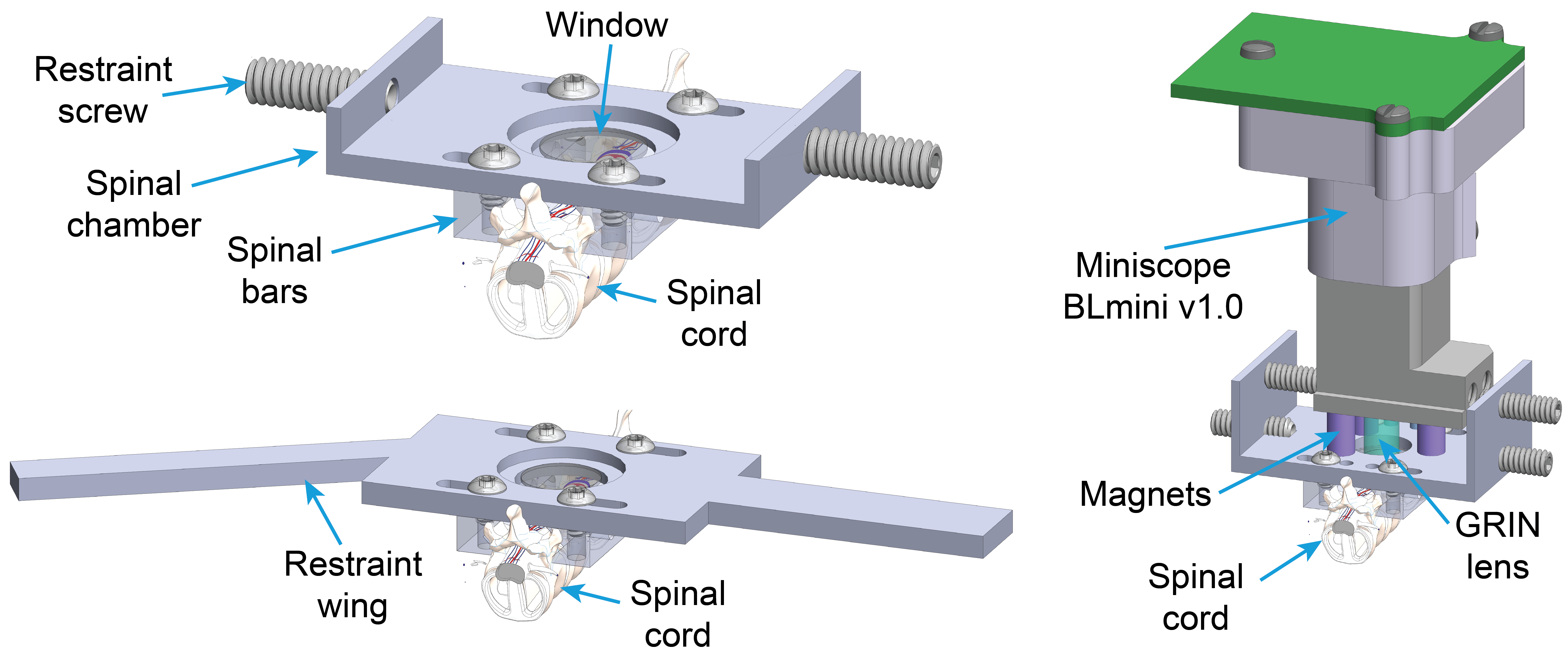
Related Publications:
Neuromodulation therapy with Individualized Network Targeting in Depression
Anusha Allawala , Yunshu Fan , Stephanie Vartany , Michelle Akerman | Collaborators: Baylor College of Medicine, UCLA, Boston Scientific
Major Depressive Disorder (MDD) is the most common mental disorder in the US and the leading source of disability. Despite multiple treatment options, up to one-third of patients are diagnosed as having Treatment-Resistant Depression (TRD). While early clinical trials of Deep Brain Stimulation (DBS) for depression have shown promise, results from prior studies have been mixed due to a large number of factors, including the lack of understanding surrounding the disruption of circuitry in depression, and the mechanisms by which DBS perturbs these circuits. Additionally, TRD encompasses a wide variety of symptoms that vary within and across patients. We are using multi-modal approaches to individualize DBS therapy for patients with depression using multi-site DBS and intracranial recordings. Our goal is to ultimately tailor treatment for each patient depending on their network activity and the nature and severity of behavioral impairment.
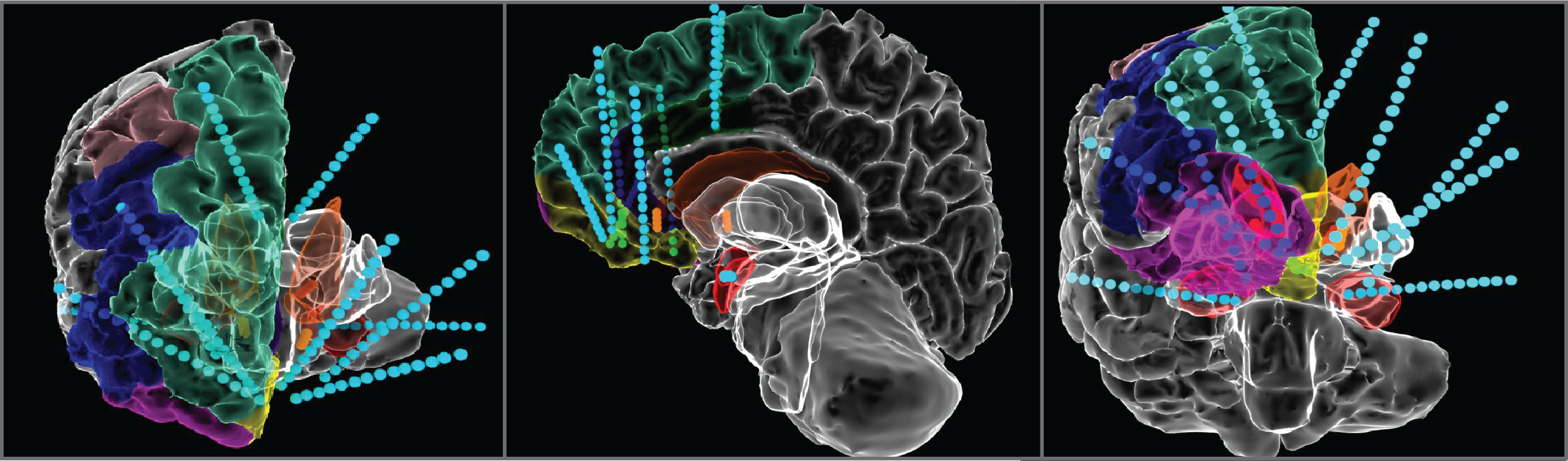
Related Publications:
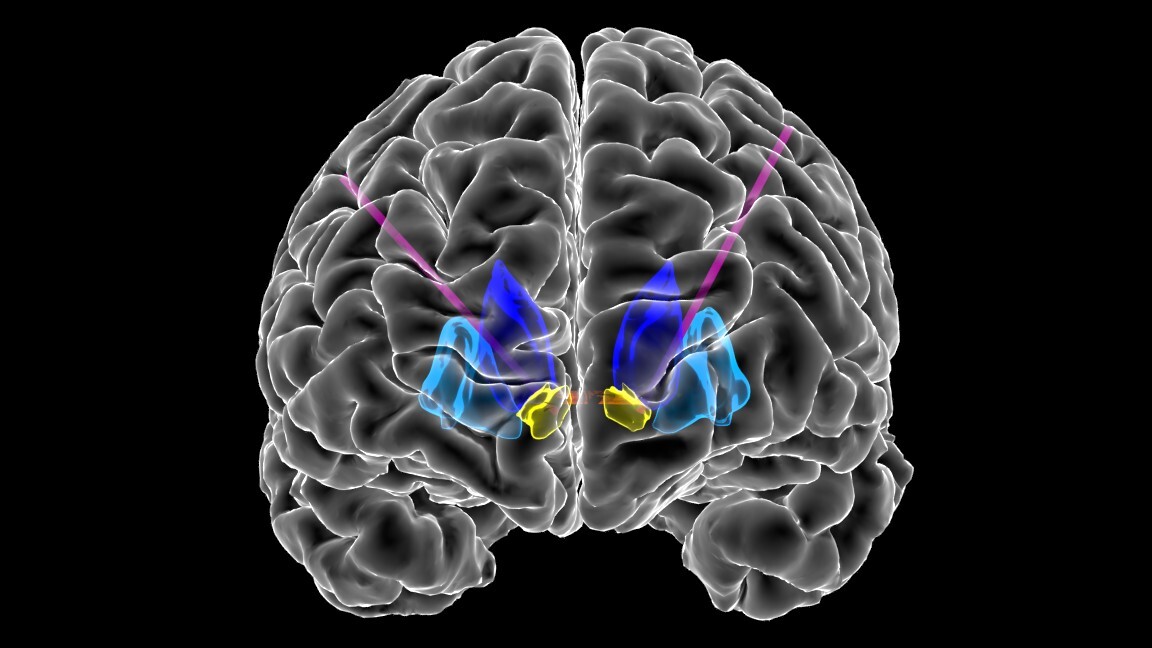
Responsive neuromodulation in Neuropsychiatric Illness
Ayan Waite , Michaela Alarie , Yunshu Fan | Collaborators: Baylor College of Medicine, Draper, Medtronic, University of Pittsburgh
Obsessive Compulsive Disorder (OCD) is a psychiatric illness marked by obsessions (recurrent unwanted or distressing thoughts) and compulsions (repetitive, ritualistic behaviors). OCD affects ~2% of the US population, and 10-20% of cases are treatment resistant. Deep Brain Stimulation (DBS) in the ventral capsule/ventral striatum (VC/VS) has been found to improve symptoms in approximately 50-70% of patients. While early trials of DBS have been promising, clinical trials have failed to date. These failures may be attributed to the “open-loop” nature of DBS, where stimulation parameters are chosen during infrequent visits to the clinician’s office. Further, the continuous stimulation fails to address the dynamic nature of OCD; symptoms often fluctuate over minutes to days. Titrating DBS to respond to symptoms as they arise (i.e. “Adaptive DBS”) may be a more effective approach for treating symptoms of OCD and reducing undesirable side effects of stimulation. We hope to design a closed-loop, adaptive system in which (1) electrodes would continuously record electrical activity from the brain, (2) recorded data would be used to classify maladaptive mental states as they arise, (3) and stimulation parameters would be adjusted accordingly to relieve symptoms.

Related Publications: